Make. Make Perfect. Repeat.™

What we do
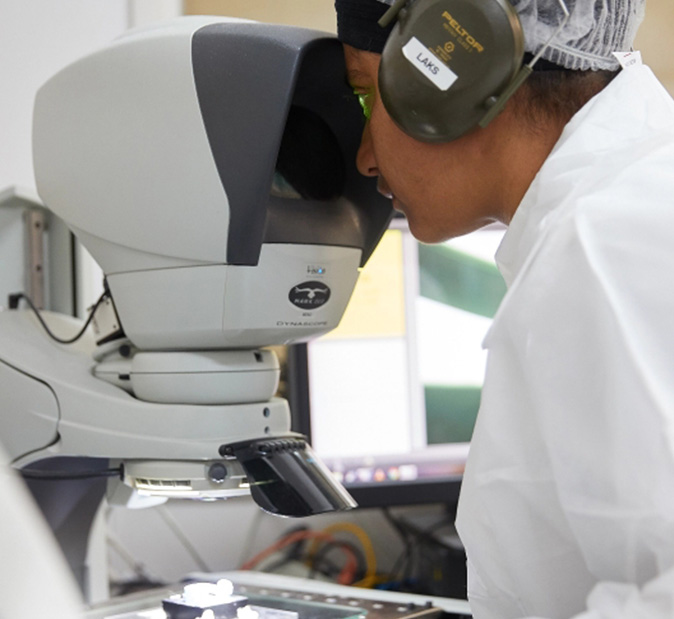
DEC partners with biotech innovation businesses to bring their devices to market. We have expertise in the development, and GMP manufacturing, of devices that deliver potent drugs to the body. We compound, mould and extruder polymer-drug composites, and silicones for both local and systemic dosing.
Our People
When a DEC engineer pauses to retool a state-of-the-art injection moulding machine, they are putting in place a small, perfectly placed, piece of a much bigger system. Learn more about our leadership, people and culture.

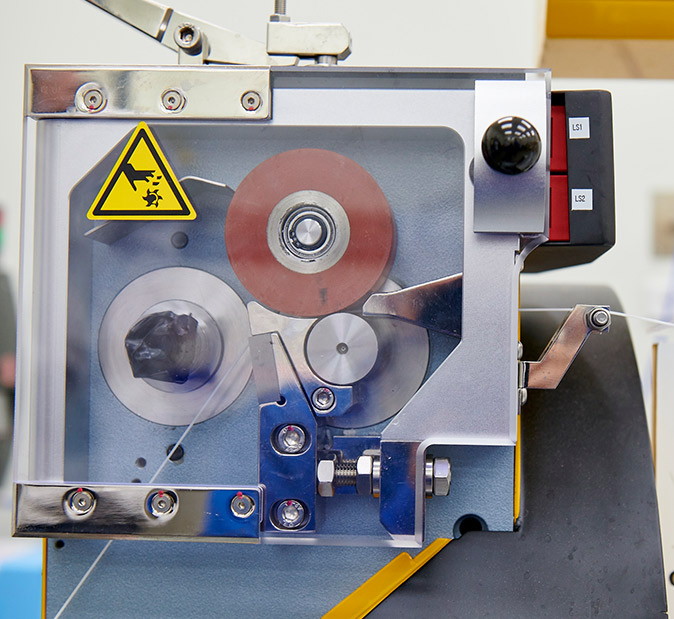
Our Strategy
+ Values
DEC is led with purpose and a strategic vision. Success is defined by our ability to ignite customers’ advancement in human and animal technologies.
History
DEC has been practising perfect since 1941. For over eighty years, DEC has been defined by the idea that innovative thinking must be met with manufacturing excellence.

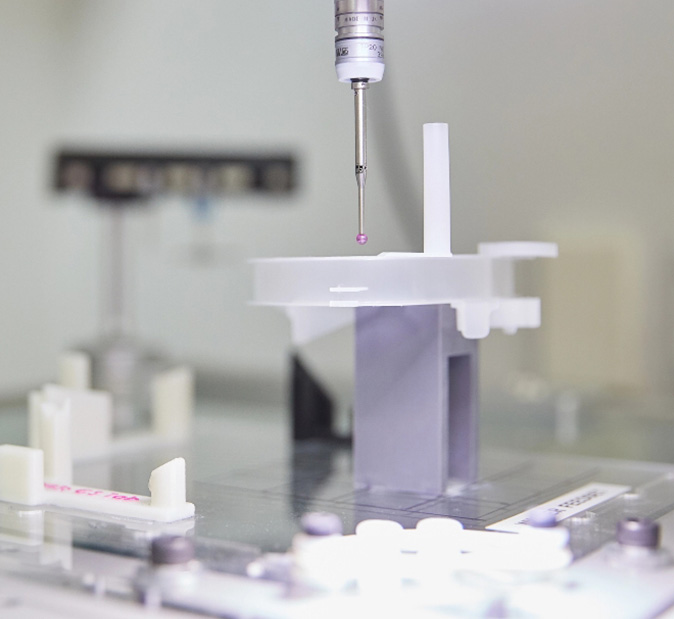
Global Markets
DEC distributes over nine million FDA-approved health devices to 57 international markets. Our links to global pharmaceutical brands, and big medical device businesses represents a worldwide network for your healthcare innovation.
Quality
DEC has achieved FDA approval into the highly-regulated US health market, and equivalent registrations (like MedSafe or TGA), assembling to ISO-13485 in our GMP facility.
We use our GMP capabilities to develop and manufacture Controlled Drug Release devices and other human and animal health products.